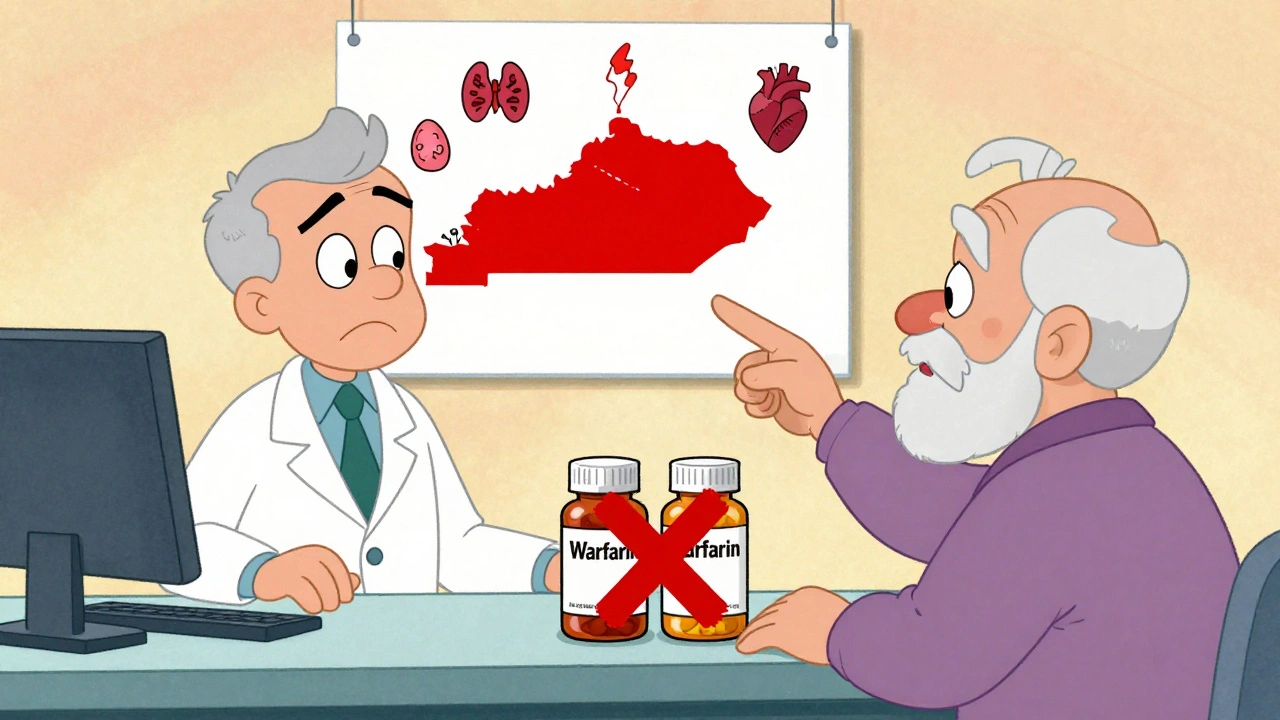
When you fill a prescription for a medication like warfarin, levothyroxine, or phenytoin, you might assume the pharmacist can swap it for a cheaper generic version without issue. But in many states, that’s not allowed-and for good reason. These are NTI drugs: Narrow Therapeutic Index medications where even tiny changes in dose can lead to serious harm. A 5% difference in blood levels might mean the difference between controlling seizures and having a seizure, or between preventing a clot and causing a stroke. The FDA says bioequivalence standards are enough for all drugs. But 27 states don’t agree. And the rules? They vary wildly.
What Exactly Are NTI Drugs?
NTI drugs are not just any strong medications. They’re ones where the gap between a safe, effective dose and a toxic or ineffective one is razor-thin. Think of it like walking a tightrope. Too little, and the drug doesn’t work. Too much, and you risk organ damage, overdose, or life-threatening side effects. Common examples include:- Warfarin (blood thinner)
- Levothyroxine (thyroid hormone)
- Phenytoin, carbamazepine (antiseizure drugs)
- Lithium (mood stabilizer)
- Digoxin (heart medication)
Why Do States Have Their Own Rules?
The FDA’s position since 1997 has been that the 80-125% bioequivalence range (meaning a generic can be up to 20% stronger or weaker than the brand) is safe for all drugs. But doctors and pharmacists who treat patients on these medications know better. A 2023 meta-analysis found that over one-third of patients stabilized on brand-name levothyroxine saw their thyroid hormone levels shift after switching to a generic. That’s not a minor fluctuation-it often means adjusting doses, extra lab tests, and months of instability. States stepped in because patients were getting hurt. A 2022 study showed states with NTI substitution bans saw 18.7% fewer adverse events tied to warfarin. That sounds small-just a 0.3% absolute drop-but for someone on blood thinners, that one person could be your neighbor, your parent, or you.How States Differ: From Strict Bans to Recommendations
There’s no national standard. Instead, you’ve got a patchwork of approaches:- Kentucky and Pennsylvania: These states maintain official lists of NTI drugs that cannot be substituted under any circumstance. If your prescription is for warfarin or digoxin, the pharmacist must give you the exact brand or generic specified-no swaps.
- South Carolina: They don’t ban substitution outright. Instead, they recommend against it for NTI drugs, plus specific brands like Synthroid and Premarin, and other ‘critical drugs’ like insulin and asthma inhalers. Pharmacists can still substitute, but they’re expected to know the risks.
- Tennessee: Allows substitution of A-rated generics, but makes one clear exception: antiepileptic drugs for patients with epilepsy or seizures. Even then, if the doctor says ‘dispense as written,’ the pharmacist must follow.
- California: Defines ‘critical dose drugs’ as those where a 10% or less change in blood concentration can be dangerous. Pharmacists must notify the prescriber whenever they substitute one of these drugs.
- Iowa: Doesn’t have its own NTI list. Pharmacists are told to rely solely on the FDA’s Orange Book ratings. If it’s rated ‘A,’ they can substitute.

What Happens When You Switch Brands?
Switching from brand to generic-or even between different generics-can cause problems even if the drug is technically ‘bioequivalent.’ Why? Because NTI drugs are absorbed, metabolized, and cleared from the body differently depending on the formulation. A patient on brand-name Synthroid for years might feel fine. Switch to a generic, and suddenly their TSH levels spike. They get fatigued, gain weight, or worse-develop heart rhythm problems. Re-stabilizing can take months. A 2023 American College of Clinical Pharmacy report reviewed 17 studies and found that 32.4% of patients on levothyroxine needed a dose adjustment after switching generics. That’s more than one in three. And it’s not just about labs. Patients report feeling worse-brain fog, anxiety, palpitations-long before their blood tests change.The Bigger Picture: Biosimilars and Administrative Chaos
The problem isn’t just generics. Forty-eight states plus D.C. have laws governing biosimilar substitution-newer, complex biologic drugs like insulin or rheumatoid arthritis treatments. Now pharmacists have to juggle two sets of rules: one for small-molecule NTI drugs, another for biologics. It’s a mess. Pharmacy benefit managers (PBMs) like Express Scripts say NTI substitution restrictions increased their administrative costs by 5.7% compared to regular generics. Why? Because they have to track which states ban what, update formularies, and handle exceptions. Prescribers have to write more ‘do not substitute’ notes. Pharmacies need extra training. Patients get confused.
Is There a Fix Coming?
Yes. In January 2024, the National Association of Boards of Pharmacy introduced the Model State NTI Substitution Act. It proposes a single, evidence-based list of NTI drugs that states can adopt. Twelve have already introduced it as legislation. At the same time, the FDA announced in September 2024 that it’s reconsidering its 27-year-old stance after a Government Accountability Office report found nearly 3,000 adverse events linked to NTI drug substitutions between 2019 and 2023. Industry analysts predict that by 2027, 38 states will have moved toward standardized rules. That could cut prescription errors by over 20%. But it might also reduce generic use for NTI drugs by 8 percentage points-meaning more patients pay full price for brand-name versions.What Should You Do?
If you take an NTI drug:- Check your state’s pharmacy board website for their NTI drug list.
- Ask your pharmacist: ‘Is this substitution allowed for my medication?’
- Make sure your doctor writes ‘dispense as written’ on the prescription if you’re stable on a specific brand or generic.
- Don’t assume a generic is interchangeable-even if it’s labeled ‘A’ rated.
- Track how you feel after any switch. Fatigue, dizziness, mood changes, or irregular heartbeat? Call your doctor.
Are all generic drugs unsafe for NTI medications?
No. Many patients successfully use generic versions of NTI drugs without issues. But the risk is higher than with other medications. The problem isn’t that generics are bad-it’s that the FDA’s bioequivalence standards allow for a 20% variation in absorption, which can be too wide for drugs like warfarin or levothyroxine. If you’re stable on a specific brand or generic, switching can cause instability.
Can a pharmacist substitute an NTI drug if the doctor doesn’t say ‘do not substitute’?
It depends on the state. In states like Kentucky and Pennsylvania, pharmacists cannot substitute NTI drugs regardless of what the doctor writes. In states like Iowa, they can substitute if the FDA rates it ‘A.’ In California, they must notify the prescriber even if substitution is allowed. Always check your state’s laws-don’t assume silence means permission.
Why doesn’t the FDA just label NTI drugs officially?
The FDA has maintained since 1997 that the current bioequivalence standards are sufficient for all drugs, including those with narrow therapeutic indices. They argue that the 80-125% range is scientifically sound and that adding special rules would create unnecessary complexity. Critics say this ignores real-world patient outcomes, especially for sensitive populations like the elderly or those with chronic conditions.
What should I do if I notice side effects after switching my NTI medication?
Contact your doctor immediately. Don’t wait for your next scheduled blood test. Symptoms like unusual fatigue, heart palpitations, mood swings, or seizures could signal a dangerous change in drug levels. Bring your prescription bottle and tell your doctor exactly what you switched from and to. Many doctors will switch you back and write ‘dispense as written’ on future prescriptions.
Do insurance companies cover brand-name NTI drugs if generics are banned?
Yes, in most cases. If your state prohibits substitution, insurers are required to cover the brand-name drug without requiring prior authorization-unless the prescriber chooses a generic. Some states even mandate that insurers pay the same copay for brand and generic NTI drugs to avoid financial barriers to staying on the right version.